Unpacking The CN- Lewis Structure: A Look At Cyanide's Atomic Arrangement
Have you ever wondered what keeps molecules together, how atoms share their tiny electron bits, or why some particles behave the way they do in a chemical reaction? It's a bit like figuring out the blueprint of a building, really. For chemists, one of the first big steps in understanding these molecular blueprints involves something called a Lewis structure. It's a simple drawing, yet it tells us so much about how atoms connect and where their electrons hang out. Today, we're going to take a closer look at a very interesting and common ion: the cyanide ion, often written as CN-.
So, why is the CN- Lewis structure something worth exploring? Well, it's a fundamental building block in chemistry, and getting a good grasp on its structure helps us see why it acts the way it does. For instance, my text mentions that when CN- acts as a nucleophile, its "HOMO" attacks an "electrophilic acceptor." That might sound a bit technical, but what it really means is that the way its electrons are arranged makes it good at finding and grabbing onto other atoms in reactions. Knowing its Lewis structure is the very first step to making sense of that.
Understanding these structures, you know, is pretty important. It's not just about drawing lines and dots; it's about seeing the hidden dance of electrons that makes chemistry happen all around us, every single day. Just like how knowing the difference between a .com and a .cn domain tells you something about a website's origin—one is for commercial use, the other is China's top-level domain, as my text points out—knowing a molecule's Lewis structure tells you about its identity and behavior. It's all about distinct meanings, you see, and what those meanings allow something to do.
Table of Contents
- What is a Lewis Structure, Anyway?
- Getting Started with CN-: Valence Electrons
- Step-by-Step: Drawing the CN- Lewis Structure
- Why the CN- Lewis Structure Matters
- Common Questions About CN- Lewis Structures
- Practice Makes Perfect!
What is a Lewis Structure, Anyway?
A Lewis structure, sometimes called an electron dot structure, is a way to show the bonding between atoms of a molecule and the lone pairs of electrons that might be present. It helps us visualize the arrangement of valence electrons, which are the electrons in the outermost shell of an atom, the ones that get involved in forming chemical connections. It's a simple, yet very powerful tool, you know, that helps chemists predict a molecule's shape and how it might react.
The whole idea comes from Gilbert N. Lewis, who proposed this method to better picture molecules. It's almost like a simplified map of where all the important electrons are located. This map, so to speak, helps us understand how atoms achieve stability, usually by getting a full outer shell of eight electrons, a rule often called the octet rule. For smaller atoms, especially hydrogen, it's a duet rule, meaning two electrons are enough for stability. This drawing method is really quite clever, and it's a first step for many, many chemical ideas.
Getting Started with CN-: Valence Electrons
Before we can draw any Lewis structure, we first need to know how many valence electrons each atom brings to the table. These are the electrons that are ready to make connections with other atoms. For the cyanide ion, CN-, we have two different kinds of atoms: carbon (C) and nitrogen (N). And, too, we have to remember that little minus sign, which means an extra electron is floating around, ready to join the party.
Carbon, typically, is in group 14 of the periodic table, so it has four valence electrons. Nitrogen, on the other hand, is in group 15, meaning it has five valence electrons. So, just from the atoms themselves, we're looking at a total of 4 + 5 = 9 electrons. But wait, there's that negative charge! That negative charge means the ion has gained one extra electron. So, the total number of valence electrons for the CN- ion is actually 9 + 1 = 10 electrons. That's a very important number to keep in mind, as it's the total number of dots and lines we'll be using in our drawing.
Step-by-Step: Drawing the CN- Lewis Structure
Now that we know how many electrons we're working with, let's go through the process of drawing the Lewis structure for CN-. It's a bit like following a recipe, really, with each step building on the last. We want to make sure every atom ends up happy, meaning they usually have a full outer shell of electrons.
Counting All the Electrons
As we just figured out, the cyanide ion, CN-, has a total of 10 valence electrons. We get this by taking the valence electrons from carbon (4) and nitrogen (5), and then adding one more for the negative charge. This sum, 10, is that, the total number of electrons we will distribute in our structure. It's a crucial starting point, as we can't add or take away any electrons once we begin drawing.
Connecting the Atoms
For a simple diatomic ion like CN-, there's only one way to connect the atoms: a single bond between the carbon and the nitrogen. We represent this with a single line, which stands for two shared electrons. So, we draw C-N. This uses up two of our 10 total electrons. That leaves us with 8 electrons remaining to place around the atoms. It's a straightforward start, basically, connecting the two parts.
Filling Up the Outer Shells
Next, we distribute the remaining electrons as lone pairs, making sure to satisfy the octet rule for each atom, if possible. We usually start by giving electrons to the more electronegative atom first. Nitrogen is more electronegative than carbon, so we'll start with nitrogen. Nitrogen already has two electrons from the single bond. To get to an octet (8 electrons), it needs six more. So, we place three lone pairs (6 electrons) around the nitrogen atom. Now, nitrogen has 2 (from bond) + 6 (lone pairs) = 8 electrons, which is a full octet. We have used 6 of our remaining 8 electrons, leaving us with 2 electrons.
Now, we look at carbon. Carbon only has two electrons from the single bond, and we have 2 electrons left to place. So, we put those two electrons as a lone pair on the carbon atom. At this point, carbon has 2 (from bond) + 2 (lone pair) = 4 electrons. This is not an octet. This is where the next step becomes very important, you know, to make sure everyone is stable.
Checking for Octets and Multiple Bonds
Since carbon does not have a full octet yet (it only has 4 electrons), we need to move some lone pair electrons from the nitrogen to form more bonds between carbon and nitrogen. Each lone pair moved becomes another shared bond. Nitrogen has three lone pairs. If we move one lone pair from nitrogen to form a double bond, carbon will then have 2 (from first bond) + 2 (from new bond) + 2 (lone pair) = 6 electrons. Still not an octet for carbon. So, we need to move another lone pair from nitrogen. If we move a second lone pair from nitrogen, we form a triple bond between C and N. Now, nitrogen has one lone pair left, and the triple bond (6 electrons). So, nitrogen has 6 (from bond) + 2 (lone pair) = 8 electrons. Carbon also has 6 (from bond) + 2 (lone pair) = 8 electrons. Both atoms now have a full octet! This is the most stable arrangement, more or less.
So, the final structure shows a triple bond between carbon and nitrogen, with one lone pair on carbon and one lone pair on nitrogen. It looks like C≡N, with dots on both the C and N for their lone pairs. This is pretty much the correct Lewis structure for CN-.
Figuring Out Formal Charges
To confirm our structure and find out where the negative charge on the ion actually sits, we calculate formal charges. Formal charge helps us figure out the electron distribution within a molecule. It's like a way to check if our electron sharing is fair. The formula for formal charge is: (Valence electrons) - (Non-bonding electrons) - (1/2 * Bonding electrons).
For carbon in CN- (with a triple bond and one lone pair): Valence electrons = 4 Non-bonding electrons (lone pair) = 2 Bonding electrons (triple bond) = 6 Formal charge on C = 4 - 2 - (1/2 * 6) = 4 - 2 - 3 = -1
For nitrogen in CN- (with a triple bond and one lone pair): Valence electrons = 5 Non-bonding electrons (lone pair) = 2 Bonding electrons (triple bond) = 6 Formal charge on N = 5 - 2 - (1/2 * 6) = 5 - 2 - 3 = 0
The sum of the formal charges (-1 + 0 = -1) equals the overall charge of the ion, which is -1. This confirms our Lewis structure is correct. The negative charge is located on the carbon atom, which is a bit unusual since nitrogen is more electronegative, but that's what the formal charge calculation tells us. It's a very useful check, you know, to make sure everything adds up.
Why the CN- Lewis Structure Matters
Understanding the CN- Lewis structure is more than just a classroom exercise; it's a key to unlocking its chemical behavior. Because the carbon atom carries that negative formal charge and has a lone pair of electrons, it makes CN- a very good nucleophile. This means it's really good at seeking out and attaching to positively charged or electron-deficient parts of other molecules. My text mentions that when CN- acts as a nucleophile, its "HOMO" (highest occupied molecular orbital) attacks an "electrophilic acceptor." This is just a fancy way of saying that the electron-rich parts of the CN- ion, particularly the lone pair on carbon, are ready to form new bonds. This reactivity is why cyanide is so important in organic synthesis, for instance, allowing us to build more complex molecules.
The triple bond also gives the CN- ion a very stable and linear shape. This linearity, along with the electron distribution, affects how it interacts with its surroundings. It's a bit like knowing the structure of a specific type of building helps you understand its function. Similarly, knowing the Lewis structure helps us predict how CN- will behave in different chemical environments. It's a really foundational piece of knowledge, actually, that opens up so many other ideas in chemistry.
This kind of structural insight is also why we draw Lewis structures for other related species, as my text implies with its mention of drawing structures for CN and CN+. Each small change in electrons makes a big difference in how the molecule looks and acts. For example, if you were to draw the Lewis structure for neutral CN, you'd find it has an odd number of electrons, making it a radical, which behaves quite differently. So, seeing the electron setup really does tell a story.
Common Questions About CN- Lewis Structures
People often have a few questions when they're first getting to grips with the CN- Lewis structure. Let's tackle some of the common ones, because, you know, it helps to clear things up right away.
How many valence electrons does the cyanide ion (CN-) have?
The cyanide ion, CN-, has a total of 10 valence electrons. This comes from 4 valence electrons from carbon, 5 from nitrogen, and an additional 1 electron because of the negative charge on the ion. So, 4 + 5 + 1 equals 10. That's the count we use for all the bonds and lone pairs.
How many bonds are present in its Lewis structure?
In the most stable Lewis structure for the cyanide ion, CN-, there are three bonds between the carbon and nitrogen atoms. This is a triple bond. This triple bond helps both the carbon and nitrogen atoms achieve a full octet of electrons, making the structure very stable. It's a pretty strong connection, that, between the two atoms.
How do you draw the Lewis structure for CN-, adding charges and lone electron pairs to the appropriate atoms?
To draw the Lewis structure for CN-, you first count the total valence electrons (10). Then, you connect carbon and nitrogen with a single bond. After that, you distribute the remaining electrons as lone pairs to give each atom an octet, starting with the more electronegative atom (nitrogen). You'll find you need to convert some lone pairs into additional bonds to give both atoms octets, resulting in a triple bond between C and N. Finally, calculate formal charges to place the overall negative charge on the carbon atom. You'll show one lone pair on carbon and one lone pair on nitrogen, along with the triple bond. Learn more about fundamental chemistry concepts on our site, and you can also link to this page for more in-depth explanations of drawing Lewis structures.
Practice Makes Perfect!
Drawing Lewis structures, just like anything else, gets easier with practice. The more you draw, the more you start to see the patterns and understand why atoms behave the way they do. It's a really rewarding skill to develop, because it helps you visualize things that are otherwise invisible. So, keep practicing with different molecules and ions.
Thinking about how CN- acts as a nucleophile, as mentioned in my text, is a great way to see how these simple drawings connect to real chemical reactions. The structure isn't just static; it tells us about potential for movement and change. It's a bit like how different domain names, like .com.cn or .org.cn, have special meanings and uses, as my text points out. Each structure, whether it's a domain or a molecule, has its own rules and possibilities. Keep exploring, and you'll find chemistry is full of fascinating connections!
Lewis Structure Calculator

Draw Lewis Structure For Cn+
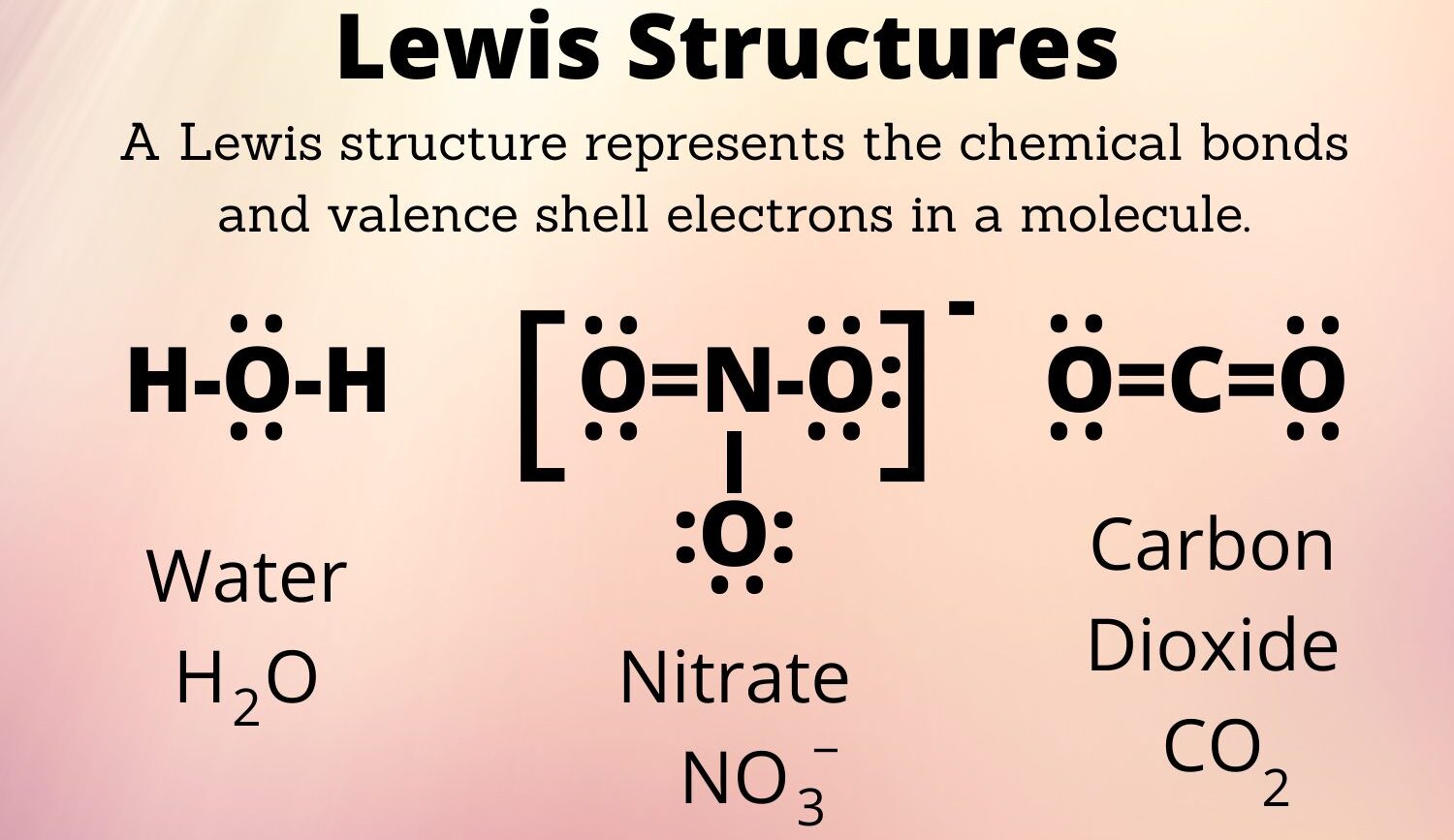
Lewis Structure