Understanding N2 Atomic Weight: More Than Just A Number
When we talk about "N2," it's interesting how many different things can pop into people's minds. For some, it might bring up images of thrilling track days, the excitement of safe and structured riding events, or maybe even the community spirit of a club that truly knows road racing in America. In fact, if you've been around certain circles, you'd know N2 as a club that, you know, really dominates the entire east coast, offering programs like advanced training and special packages for memberships, which, by the way, are often available around this time of year, like those N2 2025 packages that went on sale back in October, or the 2024 ones that wrapped up in December.
Yet, for others, N2 points to something completely different, something very fundamental to our existence. This other N2 is not about motorcycles or racing, but rather about a tiny, invisible molecule that makes up a huge part of the air we breathe every single day. It's a key player in countless natural processes and industrial applications, and its properties, including its atomic weight, are quite important.
So, today, we're going to explore this other N2, the one that scientists and curious minds often think about when they hear the term. We'll look at what its "atomic weight" actually means, how we figure it out, and why this seemingly simple number has such a big impact on our world. It's a bit of a journey into the very building blocks of matter, you see, and it’s pretty cool how it all works.
Table of Contents
- What is N2? The Molecule in Our Air
- Atomic Weight vs. Molecular Weight: Getting Our Terms Straight
- The Building Block: Nitrogen's Atomic Weight
- Isotopes of Nitrogen: A Slight Variation
- Calculating N2's Molecular Weight: A Simple Sum
- Why Does N2's Molecular Weight Matter? Its Role in Life and Industry
- N2 in Everyday Moments: More Than Just a Number
- The Periodic Table's Story: Where Nitrogen Lives
- A Look Back: How We Figured This Out
- Frequently Asked Questions About N2 Atomic Weight
- Wrapping Up: The Significance of N2
What is N2? The Molecule in Our Air
N2, in the context of chemistry, stands for dinitrogen. This is a molecule made up of two nitrogen atoms held together by a very strong triple bond. It’s a gas at room temperature and, actually, it's the most abundant gas in Earth's atmosphere, making up about 78% of the air we breathe. You know, it's pretty incredible when you think about it.
This molecule is quite stable and, in a way, it’s not super reactive under normal conditions. This stability is due to that strong triple bond between the two nitrogen atoms. Because of this, N2 gas is often used when you need an inert, or non-reactive, atmosphere for various processes, like keeping food fresh or protecting sensitive electronics during manufacturing.
So, while the term "N2" might make some folks think of the excitement of a track day, for many others, it instantly brings to mind this crucial atmospheric gas. It's a simple molecule, just two atoms, but its role in the world is anything but simple, which is rather interesting.
Atomic Weight vs. Molecular Weight: Getting Our Terms Straight
When someone mentions "n2 atomic weight," it can be a little bit confusing because, strictly speaking, N2 is a molecule, not a single atom. Atoms have atomic weights, and molecules have molecular weights. So, when we talk about "n2 atomic weight," we're really referring to the molecular weight of the N2 molecule. It’s a subtle but important distinction, you know, for clarity.
The atomic weight of an element is the average mass of the atoms of that element, taking into account the different isotopes and their natural abundance. It's usually measured in atomic mass units, or amu. For instance, a single nitrogen atom has its own atomic weight.
A molecular weight, on the other hand, is the sum of the atomic weights of all the atoms in a molecule. Since the N2 molecule has two nitrogen atoms, its molecular weight is essentially the atomic weight of nitrogen multiplied by two. So, when you hear "n2 atomic weight," it's usually a shorthand for the molecular weight of dinitrogen, which is, in fact, what we're talking about here.
The Building Block: Nitrogen's Atomic Weight
To understand the molecular weight of N2, we first need to look at the atomic weight of a single nitrogen atom. Every atom has a nucleus at its center, containing protons and neutrons, and electrons orbiting around it. The number of protons determines the element; nitrogen atoms always have seven protons.
The atomic weight of an element, like nitrogen, is essentially a measure of its mass. This mass comes primarily from the protons and neutrons in its nucleus. Electrons, you see, contribute very little to the overall mass. The atomic weight listed on the periodic table for nitrogen is about 14.007 amu. This number isn't a whole number, and that's because it's an average, taking into account the slightly different versions of nitrogen atoms that exist in nature.
So, this 14.007 amu is the average mass of a nitrogen atom as it's found naturally. It's the fundamental piece we need to put together the puzzle of the N2 molecule's weight. It's a rather precise number, too, which helps in many scientific calculations.
Isotopes of Nitrogen: A Slight Variation
You might be wondering why the atomic weight isn't a neat, whole number. This is because most elements, including nitrogen, have isotopes. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutrons means they have slightly different masses.
Nitrogen, for example, has two main stable isotopes: nitrogen-14 (14N) and nitrogen-15 (15N). Nitrogen-14 is by far the most common, making up about 99.6% of all natural nitrogen atoms. It has 7 protons and 7 neutrons. Nitrogen-15, on the other hand, has 7 protons and 8 neutrons, making it a tiny bit heavier.
The atomic weight of 14.007 amu that you see on the periodic table is a weighted average of the masses of these isotopes, based on how much of each exists naturally. So, while a single nitrogen-14 atom has a mass very close to 14 amu, and a nitrogen-15 atom very close to 15 amu, the average reflects their natural proportions. It's, like, a statistical thing, you know?
Calculating N2's Molecular Weight: A Simple Sum
Now that we know the atomic weight of a single nitrogen atom, figuring out the molecular weight of the N2 molecule is quite straightforward. Since the N2 molecule is made of two nitrogen atoms, you simply add their atomic weights together. It's a simple calculation, really.
So, if the average atomic weight of nitrogen is approximately 14.007 amu, then the molecular weight of N2 would be:
- Molecular Weight of N2 = Atomic Weight of Nitrogen + Atomic Weight of Nitrogen
- Molecular Weight of N2 = 14.007 amu + 14.007 amu
- Molecular Weight of N2 = 28.014 amu
This 28.014 amu is the average mass of an N2 molecule. This value is really important for chemists and scientists who work with gases or reactions involving nitrogen, because it helps them figure out how much of a substance they have, or how much they need for a particular process. It’s, like, a very basic but essential piece of information.
This number, 28.014 amu, is often expressed in grams per mole (g/mol) when dealing with larger quantities of the gas in a laboratory setting. A mole is just a very large number of molecules, making it easier to measure and work with in practical situations. You know, it's just a way to scale things up.
Why Does N2's Molecular Weight Matter? Its Role in Life and Industry
The molecular weight of N2, while a specific number, is incredibly important because it influences many of the properties and behaviors of nitrogen gas. These properties, in turn, affect everything from the air we breathe to vast industrial processes. It’s, in a way, a silent workhorse of the planet.
N2 in the Air We Breathe
As we mentioned, N2 makes up about 78% of our atmosphere. Its relatively low molecular weight means it's a light gas, which helps it stay well-mixed in the atmosphere. This abundance and stability mean it acts as a diluent for oxygen, preventing rapid combustion and making the air safe to breathe. If our air were just oxygen, things would, you know, burn up much faster. This balanced composition is pretty essential for life on Earth.
Biological Importance: The Nitrogen Cycle
While N2 gas itself is quite unreactive, it's a crucial part of the nitrogen cycle, which is fundamental for all living things. Plants and animals can't directly use atmospheric N2. Instead, certain bacteria "fix" nitrogen, converting it into forms like ammonia and nitrates that plants can absorb from the soil. This nitrogen then moves up the food chain, becoming a building block for proteins and DNA. So, that N2 molecular weight is, like, part of the very fabric of life, in some respects.
This cycle is a beautiful example of how a seemingly simple molecule, with its specific weight and properties, plays a central role in sustaining ecosystems. It’s a very complex dance of elements, you see, and N2 is a star performer.
Industrial Uses: From Fertilizer to Freezing
The properties of N2, which are tied to its molecular weight, make it incredibly useful in industry. One of the most significant uses is in the Haber-Bosch process, which converts atmospheric N2 into ammonia. This ammonia is then used to produce fertilizers, which are vital for growing food crops worldwide. Without this process, feeding the global population would be a much harder task, you know, truly.
Because N2 is inert, it's also used to create non-reactive atmospheres. This is important in packaging sensitive foods to prevent spoilage, in manufacturing electronics to prevent oxidation, and in fire suppression systems. Liquid nitrogen, which is N2 cooled to extremely low temperatures (around -196°C or -321°F), is used for cryogenics, like preserving biological samples, freezing foods, and even in some medical procedures. Its relatively low molecular weight helps it achieve these very low temperatures efficiently. You can learn more about elements and their properties on our site.
N2 in Everyday Moments: More Than Just a Number
Even if you don't think about "n2 atomic weight" every day, the molecule itself touches your life in countless ways. Every breath you take, for instance, is mostly N2. The food on your plate likely grew with the help of nitrogen-based fertilizers. That bag of chips might stay fresh longer because it's filled with nitrogen gas. Even some car tires are filled with nitrogen because it's less likely to leak than air and helps maintain tire pressure more consistently. It’s, like, everywhere, you know?
Understanding the basic properties of N2, including its molecular weight, helps us appreciate the intricate ways chemistry shapes our world. It's not just a number on a chart; it represents a fundamental component of our planet and a versatile tool in human innovation. And, you know, it's pretty neat how that works.
The Periodic Table's Story: Where Nitrogen Lives
Nitrogen has a special spot on the periodic table, which is, like, the chemist's ultimate cheat sheet for elements. You can find nitrogen in Group 15, which is also called the pnictogen group. It’s also in Period 2. This position tells us a lot about its behavior and how it interacts with other elements.
Being in Group 15 means nitrogen has five valence electrons, which are the electrons in its outermost shell. These are the electrons involved in forming chemical bonds. This electron configuration is why nitrogen often forms three bonds, like the triple bond in the N2 molecule, or why it can form ions with a charge of -3. Its placement, you see, really dictates a lot of its chemical personality. You can also explore more about the periodic table and its elements right here.
The periodic table, in a way, organizes elements by their atomic number and electron configuration, which directly relates to their atomic weight and how they form molecules like N2. It's a very clever system, truly, that helps us make sense of all the different elements.
A Look Back: How We Figured This Out
The concept of atomic weight, and subsequently molecular weight, has a rather interesting history. Early chemists, back in the 19th century, started trying to figure out the relative weights of different elements. John Dalton, for example, proposed that each element had a unique atomic weight
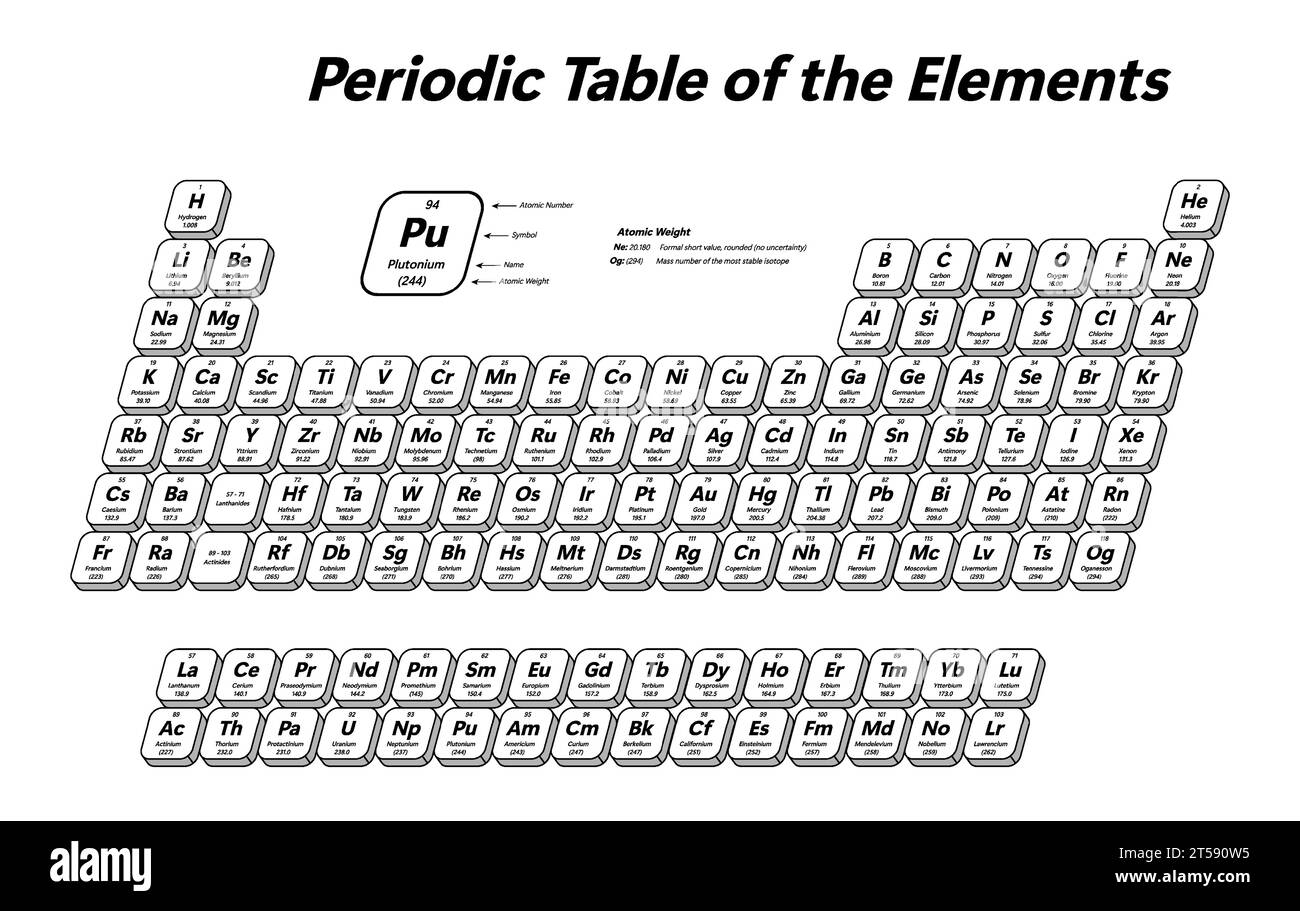
Periodic Table of the Elements - shows atomic number, symbol, name and
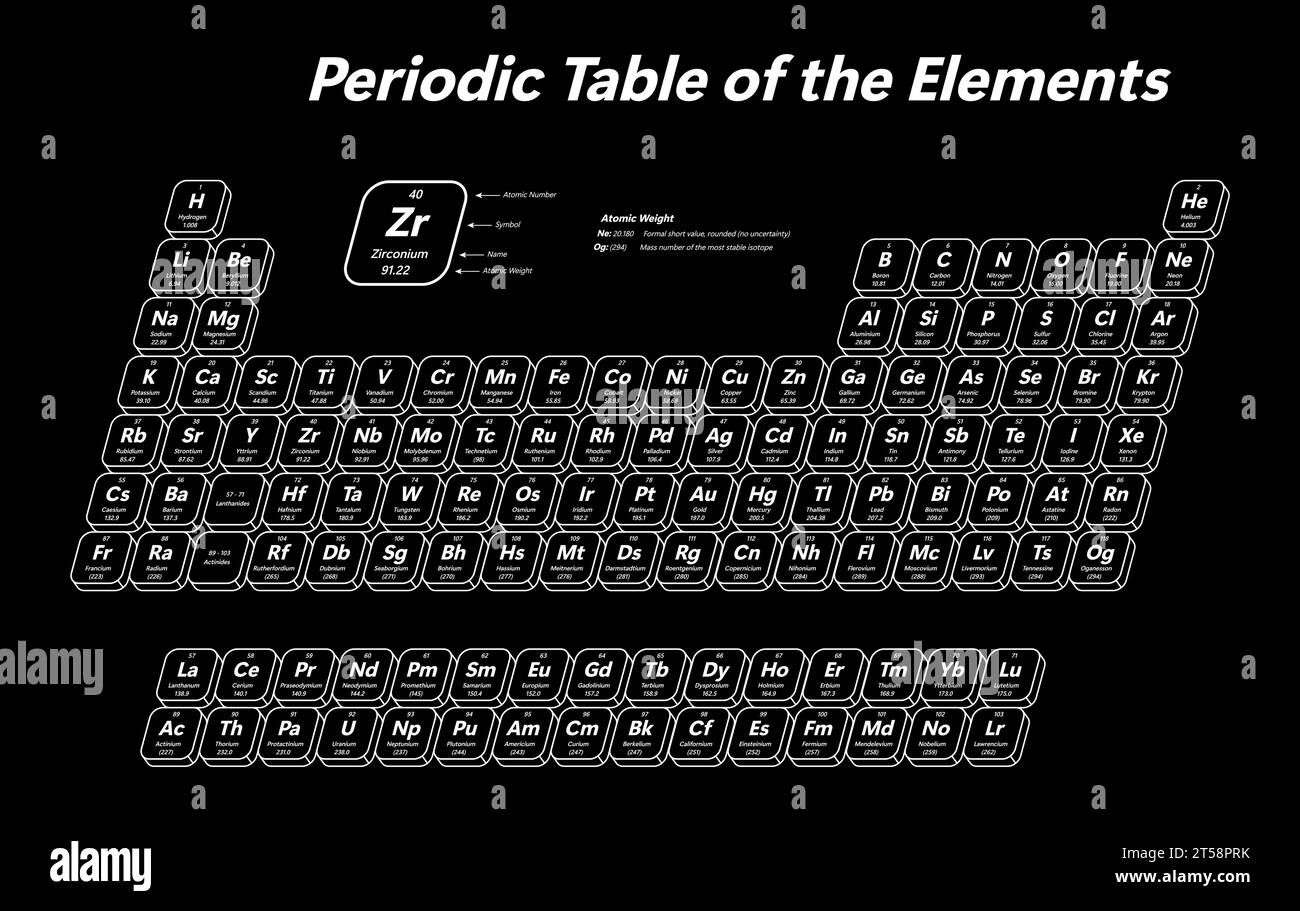
Periodic Table of the Elements - shows atomic number, symbol, name and

Atomic